Health & Fitness
8 min read
Unlocking Pediatric AML Therapy Resistance: The Splicing Mechanism
Mirage News
January 21, 2026•1 day ago
AI-Generated SummaryAuto-generated
Researchers identified sequence-dependent RNA splicing abnormalities in 36% of pediatric AML patients, linked to lower remission rates and survival. These abnormalities, driven by U2AF2 protein dysregulation rather than mutation, contribute to therapy resistance. Modulating PRMT enzymes showed potential to restore normal splicing and improve treatment response, offering a new therapeutic avenue.
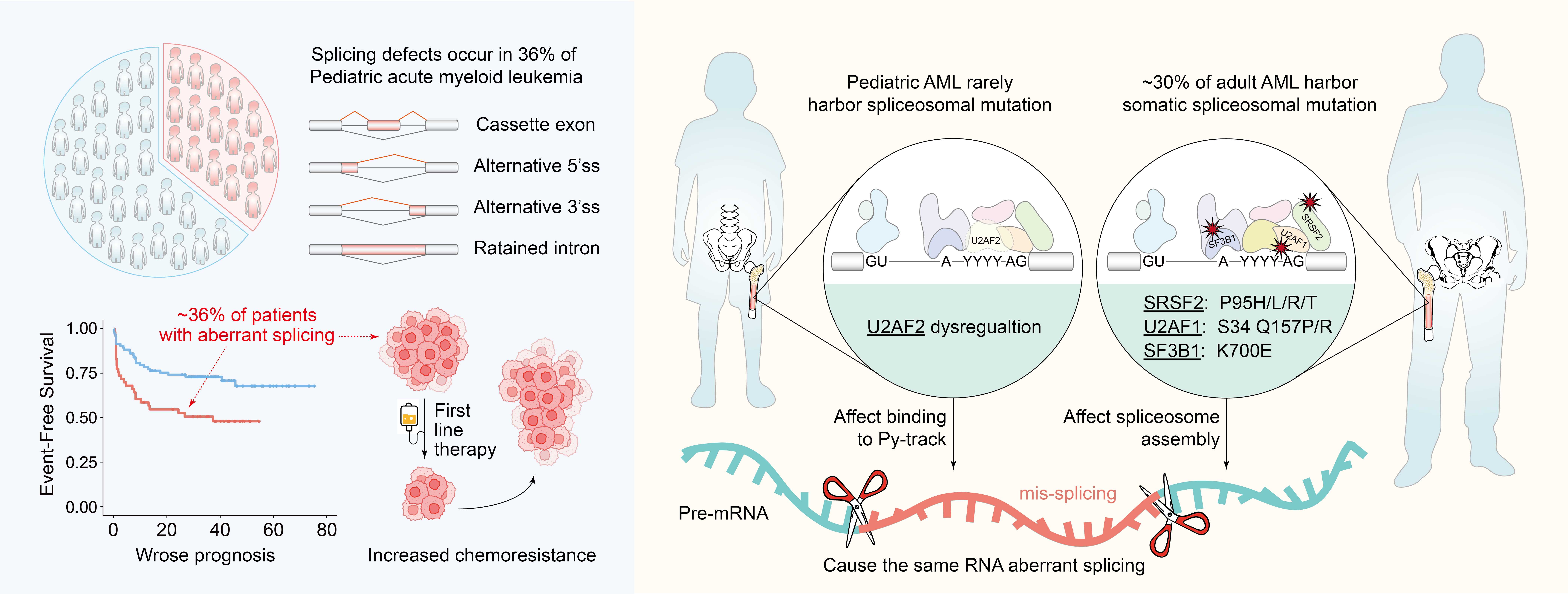
Pediatric acute myeloid leukemia (AML) is one of the most common acute leukemias in children. While treatment outcomes have improved in recent years, approximately 30% of patients relapse following initial chemotherapy and face poor survival prospects. Known resistance-associated mutations account for only a small fraction of these cases, leaving the molecular mechanisms underlying therapy resistance in most pediatric AML patients unclear.
To fill this knowledge gap, research teams led by Profs. WANG Qianfei and LIU Zhaoqi from the China National Center for Bioinformation, a research center affiliated to the Chinese Academy of Sciences, in collaboration with researchers from the Children's Hospital of Soochow University, analyzed transcriptome sequencing data from 702 pediatric AML patients. The team identified sequence-dependent ribonucleic acid (RNA) splicing abnormalities in roughly 36% of the cases. Here, "sequence-dependent" means that the RNA splicing abnormalities occurred only at certain splicing sites-specifically, those that required optimal function of a certain protein to splice correctly. The splicing abnormalities were strongly linked to lower complete remission rates and poorer overall survival-indicating a key role in disease progression and treatment response.
The findings were published in Cell Reports Medicine on January 20.
Notably, nearly 90% of these aberrant splicing events have been previously shown to promote tumorigenesis and are typically induced by splicing factor mutations in adult AML. However, such mutations are rare in pediatric AML.
Mechanistic investigations by the team revealed that functional dysregulation of the protein U2 small nuclear RNA auxiliary factor 2 (U2AF2)-rather than genetic mutation-is the primary driver of aberrant splicing in pediatric AML. These pathological splicing events preferentially occur at intronic splice sites with weak polypyrimidine tracts, making them highly sensitive to modest fluctuations in U2AF2 activity.
Importantly, pharmacological modulation of protein arginine methyltransferase (PRMT) enzymes partially restored normal splicing patterns and enhanced treatment responses in leukemia cells, pointing to a potential therapeutic strategy for overcoming drug resistance.
This study systematically defines a U2AF2-driven splicing-dysregulation-driven subtype in a large pediatric AML cohort, uncovers its link to therapy resistance, and proposes a feasible pharmacological intervention, the researchers noted.
An overview of widespread splicing dysregulation in pediatric AML. (Image by Profs. WANG Qianfei and LIU Zhaoqi's teams)
Rate this article
Login to rate this article
Comments
Please login to comment
No comments yet. Be the first to comment!
